Dr. Kerstin Müller, MA
Email: kerstin.mueller@biologie.uni-freiburg.de
Phone: ++49-761-203-2669 (office group Leubner)
Phone: ++49-761-203-2808 (lab)
Research interests:
- Seed germination
- Reactive oxygen species (ROS)
- NADH-Oxidases
- Protein oxidation
- Plant cell walls
- Plant homones
- Biomechanics of seed germination
- Early stages of seed germination
Academic career
Publications and
presentations at conferences
My postdoc project: vSEED - biomechanics of seed germination
My PhD project: Reactive Oxygen Species in seed germination
Project background
Seed model systems
Methods used in this project
Puncture-force measurement of endosperm weakening
 |
 |
 
|
 |
| |
|
My postdoc project: vSEED - biomechanics of seed germination
Project summary: The ERA-NET PG project vSEED aims at a dynamic mathematical description of germination and the development of a virtual seed combining data from different research areas.
Our task in Freiburg within the vSEED project is to investigate the biomechanics of Lepidium sativum (cress) and Arabidopsis seed germination. This includes the establishments of methods and measurements of
• puncture-force of micropylar endosperm of cress and Arabidopsis
• tensile strength of strips of the micropylar endosperm of cress
• cell extension and/or separation during radicle growth and endosperm weakening of cress and Arabidopsis
• vacuoloation and turgor pressure in the micropylar endosperm and radicle of cress and Arabidopsis
• water uptake and volume changes during the germination process of cress and Arabidopsis.
A second focus in our research is an investigation of the early stages of seed germination, that is the processes that precede and lead to testa rupture in Arabidopsis and cress.
People: Kerstin Müller as vSEED postdoc and Karin Weitbrecht as vSEED PhD student.
Collaborations: See the vSEED-webpage for further information on the project and collaborating labs.
|
|
My PhD project: Reactive Oxygen Species in seed germination
Project summary: The aim of this project is to find out whether reactive oxygen species (ROS) produced during germination are used by the seed for cell wall loosening and targeted protein oxidation. The question is to be addressed on the genetic, protein, cell wall and ROS level.
People: Kerstin Müller (PhD-student, January 2006 - July 2009).
Collaborations: Within the framework of my project, we collaborate with the following groups so far:
• Dr. Dominique Job and Claudette Job (CNRS / Bayer CropScience Joint Laboratory, Lyon, France) - Cress proteome
• Prof. Stephen Fry and Dr. Robert Vreeburg (The Edinburgh Cell Wall Group, The University of Edinburgh, UK) - Cell wall analysis
• PD Dr. Anja Krieger-Liszkay (CEA, Saclay, France) – Reactive oxygen species
• UD Dr. Ilse Kranner (Millennium Seed Bank, Kew Botanic Gardens, UK) – Reactive oxygen species
• Dr. Bill Finch-Savage and Dr. Karl Morris (Warwick HRI, University of Warwick, UK) – Cress transcriptome
Publications and presentations at conferences: See list below.
|
|

| |
Academic career |
 |
|
|
| Since August 2009 |
|
Postdoc in the ERA-NET PG project vSEED in Freiburg |
January 2006 - July 2009
 |
 |
PhD work: Promotion with summa cum laude on the topic of “Reactive Oxygen Species Play a Positive Role in Seed Germination and After-ripening”, Albert-Ludwigs-Universität, Freiburg. Supervisor: PD Dr. Gerhard Leubner.
 |
January - December 2005
 |
 |
Scientific research work (part time) in Gerhard Leubner’s seed biology lab on a scholarship basis.
 |
October 2000 - December 2005
 |
 |
Master of Arts Course (Magisterstudiengang) Scandinavian Studies and Historical Anthropology at the Albert-Ludwigs-University, Freiburg.
 |
October 1998 - December 2004
 |
 |
Master of Science Course (Diplomstudiengang) Biology (Plant Physiology, Plant Ecology and Taxonomy, Microbiology) at the Albert-Ludwigs-University, Freiburg.
 |
August - September 2004
 |
 |
Protein research (proteome analysis) in Dominique and Claudette Job’s group (Laboratoire mixte CNRS / Bayer Crop Science), Lyon.
 |
September - November 2003
 |
 |
Internship at the Millenium Seed Bank, Royal Botanic Gardens, England, supervisor: Ilse Kranner (Peter Toroop’s group).
 |
Since 2003
 |
 |
Member of the ISSS (International Seed Science Society).
 |
January 2002 - August 2003
 |
 |
Student research assistant in Gerhard Leubner’s seed biology lab.
 |
July - December 2001
 |
 |
Semester abroad at the University of Iceland (Háskóli Islands), Reykjavík.
 |
April - July 2001
 |
 |
Student teaching assistant in courses in Microbiology and Botany.
 |
 |
 |
|
|
|

| |
Project background: Seed germination, ROS, endosperm weakening
In many seeds, many economically important species among them, the endosperm is an important germination-limiting tissue barrier (see endosperm weakening webpage for details). In order for the seed to complete germination, the growth potential of the radicle must be high enough to overcome the tissue resistance of the covering layers. In the case of Arabidopsis thaliana (Arabidopsis) and Lepidium sativum (Lepidium), the resisting tissue is the endosperm layer which despite the fact that it consists of only one to two cell layers limits germination. The part of the endosperm covering the radicle tip is also called the endosperm cap. We have shown that the Lepidium endosperm cap weakens prior to endosperm rupture and that ABA delays the onset and decreases the rate of this weakening process in a dose-dependent manner (Müller et al. 2006). Figure 6 of this publication show that endosperm weakening is regulated by an ABA-gibberellin (GA) antagonism.
|
|
- Factors that influence germination, for example plant hormones, can influence the resistance of the endosperm tissue, that is promote or inhibit the cell wall weakening process which leads to endosperm weakening.
The known mechanisms for endosperm weakening include:
- Activity of hydrolases (Mannanase, ß-1,3-Glucanase,…) which cause a hydrolytic digestion of cell wall polymers
- Expansins lead to non-enzymatic loosening of cell wall hydrogen bonds
We propose a novel mechanism for endosperm weakening:
|
 |
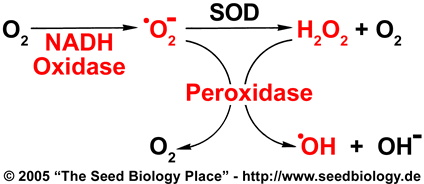
ROS produced in the way shown in this figure could play a role in endosperm weakening. In order to investigate this possibility, we chose the parts marked red in the model to focus on in our experiments: NADH-Oxidases, peroxidases, superoxide, hydrogen peroxide and hydroxyl radicals.
|
|
- Apoplastic hydroxyl radicals (•OH) are known to be able to cause cell wall weakening by cleaving polysaccharides, and have been shown to promote elongation growth in roots and seedlings (Schopfer et al. 2000). In order to be able weaken cell walls, hydroxyl radicals must be produced in the cell wall, as they are extremely short-lived and highly reactive.
- One model for apoplastic hydroxyl radical formation is described in the figure on the right and was proposed by Frahry and Schopfer (2001): NADH-Oxidases located in the plasma membrane catalyze the formation of apoplastic superoxide anions. Superoxide (•O2-) can be dismutated by the antioxidant enzyme superoxide dismutase (SOD), leading to the formation of hydrogen peroxide (H2O2) and molecular oxygen. Thus superoxide and hydrogen peroxide are both present in the apoplast. These molecules can now undergo a Fenton reaction in the presence of peroxidases which are abundant in the cell wall. This Fenton reaction leads to the formation of hydroxyl radicals (•OH). See figure on the right.
- A second possible mechanism for hydroxyl radical generation in the apoplast is by the non-enzymic oxidation of ascorbate to form Cu+ and H2O2. Again, •OH radicals are produced via the Fenton reaction. This mechanism has been postulated by Stephen Fry (1998).
|
|
 |
|
Seed model systems |
|
In this project, we combine the advantages of two model systems: We use the established system Arabidopsis thaliana (Brassicaceae) and the emerging seed model system Lepidium sativum (garden cress, Brassicaceae).
While Arabidopsis has the many advantages resulting from a sequenced genome and a large amount of available data, mutants and method, it has its limits as a seed model system has its limits. Most of these limits are due to the small size of Arabidopsis seeds. It is for example not possible to directly quantify endosperm weakening by measuring puncture force in Arabidopsis. Our solution to this problem was using a closely related species with highly similar seed anatomy and germination pattern, but with bigger seeds. We chose Lepidium sativum which is in the same Brassicaceae subfamily as Arabidopsis, but has seeds are about ten times the size of Arabidopsis seeds.
For further details on these seed model systems see the "Seed structure" and "Endosperm weakening" webpages and the Müller et al. (2006) publication.
|
 |

|
|
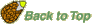
 |
|
Methods used in this project
Our methods range from physiological and biomechanical to molecular and biochemical approaches: |
|
- Seed physiology (germination curves, hormone action).
- Biomechanical measurements (puncture-force technique), for a description see below.
- Protein Analysis (Enzyme activity assays, Immunoblots, 2D-gels (in cooperation with Claudette and Dominique Jobs)).
- ROS detection (hydrogen peroxide, hydroxyl radicals, superoxide anions)
- Cell Wall Analyses (in cooperation with Robert Vreeburg and Stephen Fry).
- Molecular Analysis (RT-PCR, real-time PCR, cloning, in situ mRNA hybridization,…)
|
|
Puncture-force measurement of endosperm weakening |
|
Puncture force measurements are a useful method requiring a suitable seed system, a detailed description and discussion of the technique is published in Müller et al. (2007). The choice of the seed system for puncture force experiments strongly influences the experimental options the method offers. It is essential that the seeds are large enough to be fixed properly and leave space for the metal probe to be lowered onto the tissue with no or very little friction. In an optimal seed system for puncture force experiments it should be possible to puncture the individual seed coats (testa, endosperm) separately in order to be able to assign the weakening to a specific seed cover.
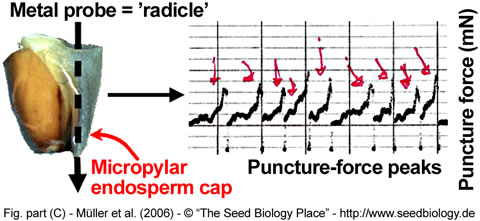
We measure puncture force of Lepidium seeds by using a custom-made machine (Figure A,B, on the right). A seed is cut in half and the radicle tip carefully removed, leaving the empty but intact endosperm cap. A metal probe shaped roughly like the radicle tip is slowly lowered into the endosperm cap. The force it takes to rupture the endosperm, the puncture force, is calculated from the size of the peak that signifies the pressure on the endosperm tissue. The lower the puncture force, the softer the tissue. (Figure C, below).
 |
 |
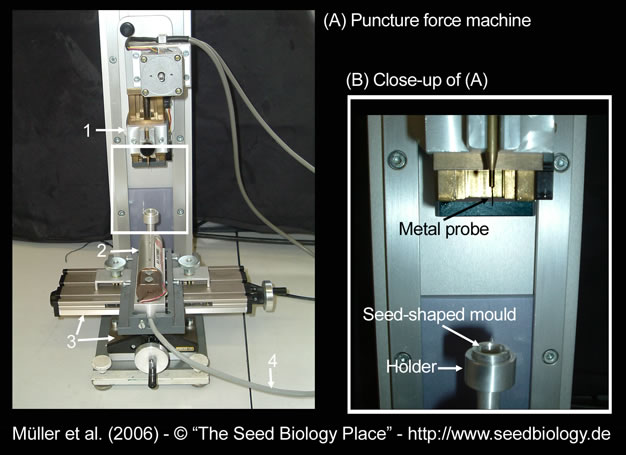
Figure. Puncture-force method for measuring endosperm weakening of Lepidium.
Müller K, Heß B, Leubner-Metzger G.
A role for reactive oxygen species in endosperm weakening.
In: Adkins S, Ashmore S, Navie S (eds). Seeds: Biology, Development and Ecology.
CAB International, Wallingford, UK. Chapter 30, pp. 287-295 (2007)
|
|
| |
 |
|
|

| |
Publications and
presentations at conferences |
 |
|
|
Peer-reviewed publications
Linkies A, Schuster-Sherpa U, Tintelnot S, Leubner-Metzger G, Müller K (2009)
Peroxidases identified in a subtractive cDNA library approach show tissue-specific transcript abundance and enzyme activity during seed germination of Lepidium sativum
Journal of Experimental Botany, in press (10.1093/jxb/erp318)
Müller K, Linkies A, Vreeburg RAM, Fry SC, Krieger-Liszkay A, Leubner-Metzger G (2009)
In vivo cell wall loosening by hydroxyl radicals during cress seed germination and elongation growth.
Plant Physiology 150: 1855-1865
Müller K, Carstens AC, Linkies A, Torres MA, Leubner-Metzger G (2009)
The NADPH-oxidase AtrbohB plays a role in Arabidopsis seed after-ripening.
New Phytologist 184: 885-897
Müller K, Job C, Belghazi M, Job D, Leubner-Metzger G (2009)
Proteomics reveal tissue-specific and hormone-induced changes in the endosperm cap proteome during cress (Lepidium sativum) seed germination
Proteomics, accepted for publication
Linkies A, Müller K, Morris K, Turecková V, Wenk M, Cadman CSC, Corbineau F, Strnad M, Lynn JR, Finch-Savage WE, Leubner-Metzger G (2009)
Ethylene interacts with abscisic acid to regulate endosperm rupture during germination; a comparative approach using Lepidium sativum (cress) and Arabidopsis thaliana
The Plant Cell, accepted for publication
Graeber, K., Linkies, A., Müller, K., Wunchova, A., Rott, A., and Leubner-Metzger, G. (2009)
Cross-species approaches to seed dormancy and germination: Conservation and biodiversity of ABA-regulated mechanisms and the Brassicaceae DOG1 genes
Plant Molecular Biology, re-submission under review
Müller K, Tintelnot S, Leubner-Metzger G (2006)
Endosperm-limited Brassicaceae seed germination: Abscisic acid inhibits embryo-induced endosperm weakening of Lepidium sativum (cress) and endosperm rupture of cress and Arabidopsis thaliana
Plant and Cell Physiology 47: 864-877
Manz B, Müller K, Kucera B, Volke F, Leubner-Metzger G (2005)
Water uptake and distribution in germinating tobacco seeds investigated in vivo by nuclear magnetic resonance imaging
Plant Physiology 138: 1538-1551
Petruzzelli L, Müller K, Hermann K, Leubner-Metzger G (2003)
Distinct expression patterns of ß-1,3-glucanases and chitinases during the germination of Solanaceous seeds
Seed Science Research 13: 139-153
Papers in conference proceedings and poster presentations
Poster (presenting author) at the ROS in Plants: SFFR Plant Oxygen Group meeting on reactive oxygen and nitrogen species, Ghent, Belgium, September 2007:
Müller K, Fry S, Vreeburg R, Leubner-Metzger G
“A Role for Reactive Oxygen Species in Seed Germination: Endosperm Weakening and Radicle Elongation”
Müller K, Heß B, Leubner-Metzger G
A role for reactive oxygen species in endosperm weakening.
In: Adkins S, Ashmore S, Navie S (eds). Seeds: Biology, Development and Ecology. CAB International, Wallingford, UK. Chapter 30, pp. 287-295 (2007)
Leubner-Metzger G, Kucera B, Müller K
Emerging and established model systems for endosperm weakening
In: Adkins S, Ashmore S, Navie S (eds). Seeds: Biology, Development and Ecology. CAB International, Wallingford, UK. Chapter 20, pp. 195-204 (2007)
Abstract and poster (presenting author) at the 3rd International Symposium on Plant Dormancy, Wageningen, The Netherlands, May 2004:
Manz B, Müller K, Hermann K, Volke F, Leubner-Metzger G
"Water Uptake and Distribution in Germinating Tobacco Seeds Investigated in Vivo by Nuclear Magnetic Resonance Microimaging".
Oral presentations
Plant ROS 2009, Helsinki, July 2009:
“A role for the NADPH-oxidase AtrbohB in seed after-ripening and germination”
9th ISSS conference on Seed Biology, Olsztyn, Poland, July 2008:
„Hydroxyl radicals and in vivo scission of cress seed cell walls“
Awarded as the best student presentation.
Millennium Seed Bank Science and Conservation Seminar Series, May 2008:
“The significance of cell wall loosening during germination of Lepidium sativum (cress) seeds“
2nd Workshop on Molecular Aspects of Seed Dormancy and Germination, Salamanca, Spain, July 2007:
“Tissue specific changes in the Lepidium sativum proteome during germination”
8th International Workshop on Seeds, Brisbane, Australia, May 2005:
“A role for reactive oxygen species in endosperm weakening”
Awarded as one of the best student presentation.
|
|
| |
 |
|
|
| |
 |
|
|
|
|
 |
| |
|
|
|

|
 |

