Dr. Krystyna Oracz,
Postdoctoral researcher until 2011
Alexander von Humboldt Fellowship
Email: krystyna_oracz@sggw.pl
New address since 2011:
Phd Krystyna Oracz Associate Professor
Department of Genetics, Breading and Plant Biotechnology and Department of Plant Physiology
Warsaw University of Life Sciences-SGGW (WULS-SGGW)
Nowoursynowska 159, building 37
Warsaw, 02-776, Poland
Tel: (+48) 22 593 25 33
My research activities cover the following aspects:
- Germination and seed dormancy -
with a focus on Reactive Oxygen Species (ROS), protein oxidation and
its interaction with hormones (i.e. ethylene).
- Cell wall modifications during endosperm weakening and embryo growth.
- Oxidative stress and antioxidant system in plants.
- Post-translational modifications of proteins (i.e. oxidation, phosphorylation)
in desiccation tolerance of resurrection plants.
- Physiological, biochemical and molecular basis of allelopathy.
Academic career
Professional activities
My Alexander von Humboldt postdoctoral project
My Claude Leon Foundation postdoctoral project
Co-supervision of PhD, Master and Diploma students
My co-tutelle PhD thesis
Peer-reviewed publications
Published contributions to academic conferences
Awards and distinctions
Symposiums and conferences
Invited seminars
 |
  |

|
 |
  |
|
Academic career |
 |
|
|
| |
|
|
Since May 2009
 |
 |
Postdoctoral researcher in the Molecular Plant Sciences, Albert Ludwigs University, Freiburg i. Br., Germany
PD Dr. Gerhard Leubner Lab
 |
March 2008 – March 2009
 |
 |
Postdoctoral researcher in the Department of Molecular and Cell Biology, University of Cape Town, South Africa
Prof. Jill Farrant Lab
 |
February 2007
 |
 |
Research visit of PhD student in Royal Botanic Gardens, Kew, United Kingdom
Supervisor: PhD Ilse Kranner
 |
November 2005 
|
 |
Research visit of PhD student in Centre National de la Recherche Scientifique, Bayer CropScience, Lyon, France
Supervisors: PhD Dominique and Claudette Job
 |
October 2003 – January 2008
 |
 |
PhD student in co-tutelle thesis in Plant Physiology Department, Warsaw University of Life Sciences (Poland) and
in EA2388 Physiologie des Semences, Université Pierre et Marie Curie, Paris 6 (France).
Thesis "Role of hydrogen cyanide in dormancy removal of sunflower (Helianthus annuus L.) embryos".
under the direction of Prof. Renata Bogatek, Prof. Francoise Corbineau, and Prof. Christophe Bailly (LPVA).
 |
January 2002 – February 2002
 |
 |
Research stage of MSc student in EA2388 Physiologie des Semences, Université Pierre et Marie Curie, Paris 6 (France).
Supervisor: Prof. Francoise Corbineau and Prof. Christophe Bailly.  |
October 1998 – July 2003
 |
 |
B.S. and M.S. in plant physiology, Plant Physiology Department, Warsaw University of Life Sciences, Poland.
Thesis “Induction of oxidative stress by sunflower phytotoxins in germinating mustard seeds”.
Supervisor: Prof. Renata Bogatek
 |
 |
 |
 |
| |
 |
|
  |
Professional activities  |
| |
 |
|
| |
 |
|
| Since 2009 |
 |
Involved in vSEED (virtual seed) - ERA-NET Plant Genomics -
European Seed Systems Biology Network Project (2009), as an associated postdoc
(the aim: reverse-genetic in cell wall loosening-related genes).
Member of Societas Humboldtiana Polonorum
 |
| 2001-2004 |
 |
Involved in scientific staff of the grant 5FP UE with acronym WECOF (QLK – CT –2000 – 01418),
“Strategies for weed control in organic farming”.
 |
| Since 2002 |
|
Member of PTBER - Polskie Towarzystwo Biologii Eksperymentalnej Roslin (Polish Society of Experimental Plant Biology)
 |
| 2001 |
|
Involved in scientific staff of the Grant from President of Warsaw University of Life Sciences,
"Involvement of oxidative stress and ABA in CN-mediated elimination of embryonic dormancy in apple” (nr.50401020011).
 |
| Since 2001 |
|
Member of ISSS (International Society for Seed Science)
 |
 |
 |
 |
 |
 |
|
|
|

| |
My Alexander von Humboldt postdoctoral project
Role of cell wall loosening in the regulation of endosperm weakening and embryo growth during Brassicaceae seed germination (Germany).
The embryo of a typical angiosperm seed is surrounded by two covering layers: the endosperm (nutritive tissue, living cells) and the testa (seed coat; maternal tissue, dead cells). The rupture of both structures is two sequential events which occur during germination of endospermic Brassicaceae seeds. The control of radicle protrusion of that type of germinating seeds is mediated, at least in part, by endosperm weakening. Lepidium sativum (‘cress’) is a member of Brassicaceae family with big seeds, and is closely related to Arabidopsis thaliana (tiny seeds). Cress seeds provide an excellent model system which can be used in experiments for studying endosperm weakening at the molecular and tissue-specific level. For the endosperm weakening, a process cell separation is required and a pectin hydrolysation has been considered to play an important role in it. The challenge of the present study is to generate transgenic Lepidium seeds altered in candidate weakening gene expression in the endosperm. This reverse genetics approach will enable me to investigate, new molecular mechanisms of endosperm weakening in germinating seeds and reach causal conclusions. The combination of direct biomechanical measurements with this reverse genetics approach, together with the transcriptome and proteome results will provide a novel insight into the role of pectin-related cell wall loosening in the regulation of embryo growth and endosperm weakening during the germination of Brassicaceae seeds.
|
|
| |
|
 |
|
My Claude Leon Foundation postdoctoral project
Post-translational modifications of proteins in desiccation tolerance of resurrection plants Xerophyta hummilis (South Africa).
|
|
Desiccation tolerance describes the ability of an organism to survive drying to equilibrium with the relative humidity of air, reviving when the water again becomes available. The seeds (orthodox seeds) of most species are desiccation tolerant (DT), but lose this tolerance when the germinating and their vegetative tissues are desiccation sensitive (DS). There are a few plants (ca 300 species of higher plants), termed resurrection plants, in which the vegetative tissues (roots and leaves) are also DT. Plants possess very efficient signal transduction pathways, controlling their cells fate, survival and function. Latest data show that posttranslational modifications (PTMs) of proteins play major roles in the regulation of various processes of plants life. The recent progress in proteomics has allowed us to considerably enlarge the role of PTMs in a complex regulatory network of desiccation tolerance. Using Xerophyta humillis (Figure 1) plants as model plant, I critically assessed current knowledge about role of PTMs of proteins in a vegetative desiccation tolerance and illustrated this knowledge with case stories wherever possible. |
 |

Figure 1. Xerophyta humilis plants: hydrated and dehydrated
(reproduced with permission from Jill Farrant)
|
|
Watch a movie: `The remarkable resurrection plant Xerophyta humilis´ (click this link)
For the reason that ROS are produced in many compartments in living cells and their increased rates during stresses conditions are observed, their involvement in signaling pathways by protein PTMs is also evoked. In this project I was focused on two PTMs issues: phosphorylation, which is well established as a widespread key regulator of signal transduction, and oxidative modifications, which from being primarily viewed as protein damage now start to emerge as crucial regulatory mechanisms in the achievement of major events of plants life.
The manuscript arising from this project is under construction. |
|
 |
|
Co-supervision of PhD, Master and Diploma students
Since April 2010: Magdalena Jura, PhD student (DFG LE720/6), co-supervision with PD Dr. Gerhard Leubner.
Project entitled: “Molecular physiology of endosperm-limited seed germination - Gene function and regulation in the endosperm of Brassicaceae species."
The mature seeds of most angiosperm species are endospermic. In many cases the micropylar endosperm that covers the radical is a mechanical constraint to embryo expansion and seed germination. Endosperm weakening is in an important trait of Brassicaceae species. Using Lepidium sativum and Arabidopsis thaliana as an experimental models, the role of pectin-related cell-wall modifications during endosperm weakening will be investigated by a cross-species reverse genetics approach and subsequent analysis of transgenic seeds by various techniques available by an interdisciplinary collaboration network.
Since August 2010: Claudia Waack, Diploma student, co-supervision with PD Dr. Gerhard Leubner.
Project entitled: “Role of pectin methylesterase (PME) during Brassicaceae seed germination."
The plant cell wall surrounding the protoplast consists of cellulose microfibrils coated by xyloglucans and embedded in a complex matrix of pectic polysaccharides. It was shown, that during germination, testa and endosperm rupture coincides with numerous modifications of the primary cell wall and degradation of the middle lamella. Using cross-species approaches will elucidate the role of pectin hydrolases i.e. pectin methylesterases (PMEs) in elongation growth and endosperm weakening of Brassicaceae seeds
Since February 2010: Dominique Jacquemoud,
Master student, internship from Lyon, France (Lab of Prof. Gilles Comte), co-supervision with PD Dr. Gerhard Leubner.
Project entitled: “Elucidation of the effect of myrigalone A on germination of Lepidium sativum seeds”
Application of allelopathic compounds as natural herbicides is a trend responding to an increasing concern about environmental care. Understanding the mechanisms implicated in such interactions could improve the use and efficiency of such alternative methods. Using Lepidium sativum seeds as a model, I currently try to reveal the possible mode of action of myrigalone A in germination, with a certain focus on the possible interaction with ROS, known as an important signaling molecule and ABA an inhibitor of germination.
|
|
 |
|
My co-tutelle PhD thesis
Regulatory role of hydrogen cyanide and ROS in dormancy removal of sunflower (Helianthus annuus L.) embryos (Poland-France). |
|
Seed dormancy and germination are very complex phenomena which involve tightly controlled signaling pathways as well as cellular and molecular regulations. In addition to hormones, there are also signaling molecules such as HCN and ROS, which seem to play important roles in seed dormancy, but whether their mechanism of action relies on a unique dominant signaling pathway or on the overlap of many is still under investigation. Freshly harvested sunflower (Helianthus annuus L.) seeds germinate poorly at temperatures below 10°C, but a short treatment by hydrogen cyanide (HCN) allows their subsequent germination at this temperature, suggesting that HCN might be an important factor in regulation of seed dormancy. Considering the role of reactive oxygen species (ROS) in various physiological processes, I have investigated whether ROS could be involved in HCN-mediated embryo dormancy removal in sunflower embryos. ROS (e.g. hydrogen peroxide) are progressively accumulated in cells of embryonic axes after HCN treatment (Figure 2). Imbibition of embryos in the presence of methylviologen (MV), a ROS generating compound, mimicked the stimulatory effect of HCN on germination, suggesting that it might improve germination through its effect on ROS metabolism and signaling.
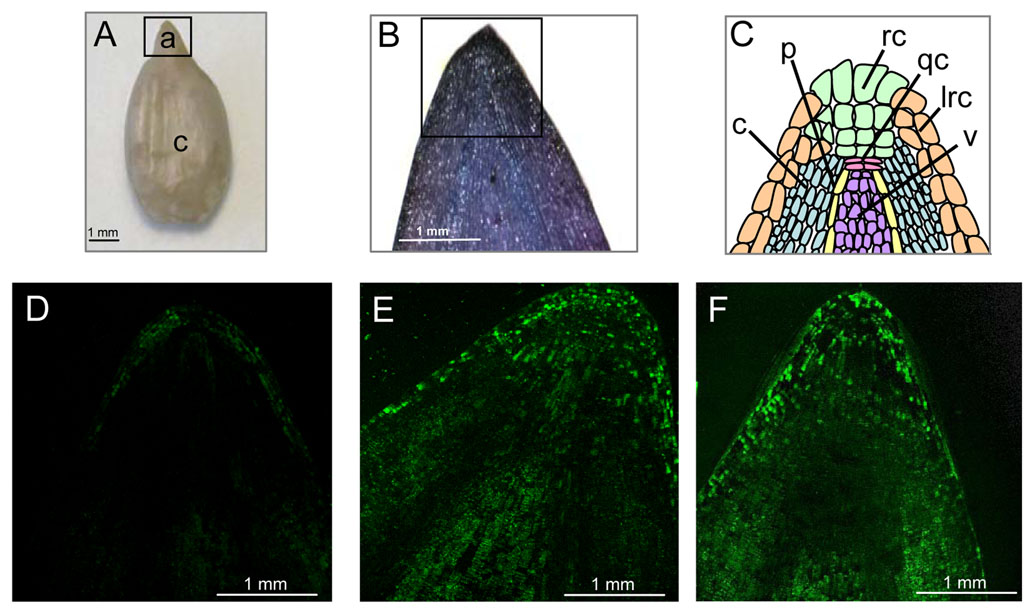
Figure 2. In situ localization of ROS production in the embryonic axes of sunflower embryos.
A, Embryo showing axe (a) and cotyledon (c; inset frame corresponds to the view shown in B).
B, Staining of embryonic axis by toluidine blue (inset frame corresponds to the views shown in confocal).
C, Schematic representation of views shown in D to F. c, Cortex; p, pericycle; rc, root cap; qc, quiescent center; lrc, lateral root cap; v, vascular.
D to F, Representative fluorescence images of sections of embryonic axes treated with DCFH-DA viewed by confocal laser scanning microscopy. Axes from dormant embryos imbibed for 24 h on water (D), from nondormant embryos imbibed for 24 h on water (E), and from dormant embryos treated for 3 h with cyanide and further imbibed on water (F; total treatment time was 24 h). (Oracz et al. 2009)
Regulation of the transcription of the main genes involved in ROS production and ROS sensing by HCN was investigated using real time RT-PCR, and demonstrated that HCN mainly acts at a post-translational level (Oracz et al. 2009). The crosstalk between HCN, ROS and ethylene in alleviation of sunflower embryo dormancy was discussed, with regards to the role of HCN and ROS in oxidation of specific proteins (Figure 3).
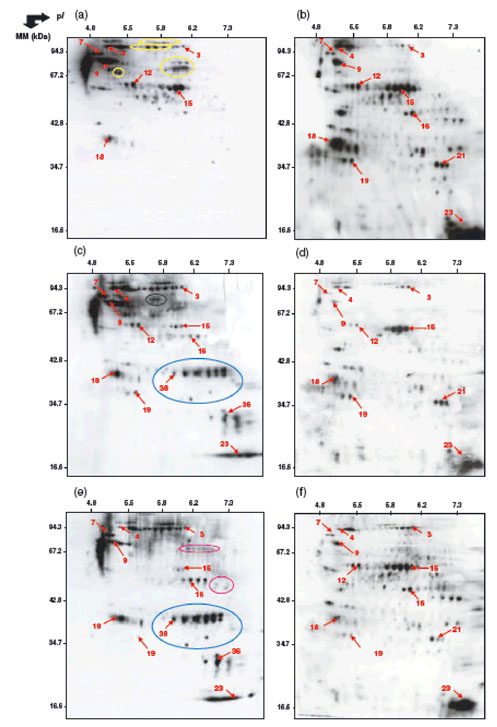 |
 |
Figure 3. Two-dimensional profiles of protein oxidation in axes of dormant and non-dormant sunflower seeds after 3 h imbibition at 10_C under various conditions.
(a) Dormant axes and (b) non-dormant axes imbibed on water; (c) dormant axes and (d) non-dormant axes imbibed in the presence of 1 mM HCN; (e) dormant axes and (f) non-dormant axes imbibed in the presence of 0.1 mM methylviologen.
Numbers indicated on the arrows correspond to proteins that have been identified by mass spectrometry (see Tables 3 and 4). Yellow circles, proteins carbonylated in dormant axes but not in non-dormant axes during imbibition on water. Blue circles, proteins specifically carbonylated in the presence of hydrogen cyanide or methylviologen. Green and purple circles, proteins specifically carbonylated in the presence of cyanide or methylviologen, respectively, during imbibition of dormant axes (Oracz et al. 2007a).
|
Obtained data allow proposing the protein oxidation as a new mechanism of action for HCN and ROS and suggesting that changes in gene expression are not the only mechanism leading to seed dormancy release (Figure 3, 4).
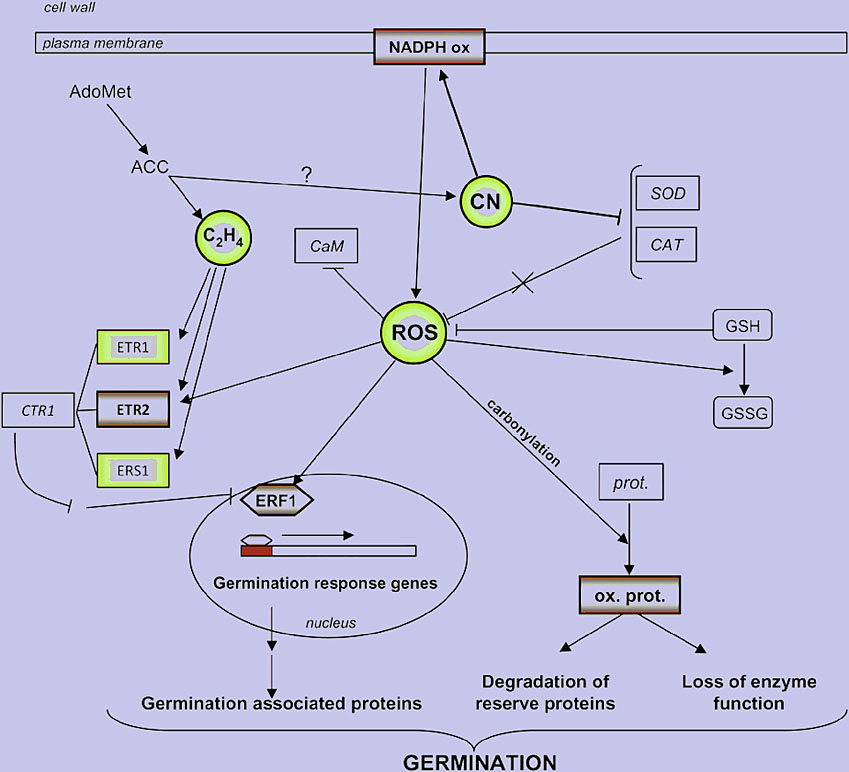
Figure 4. A scheme showing the interactions between HCN (CN in image) and ROS in dormancy alleviation, also integrating data from Oracz et al. (2007, 2008).
Positive regulators of dormancy alleviation are written in bold and negative ones in italic. ETR1, ETR2, Ethylene receptor 1 and 2, respectively; ERS1, ethylene response factor 1; CTR1, constitutive triple response 1; ERF1, ethylene response factor 1; AdoMet, S-adenosylmethionine;ACC, 1-aminocyclopropane 1-carboxylic acid; prot., protein; ox. prot., oxidized protein (Oracz et al. 2009).
Moreover, is demonstrated for the first time, that the mechanisms involved in seed dormancy alleviation by hydrogen cyanide unravel the role of reactive oxygen species as key factors of cellular signaling during germination (Figure 4).
The complete results are presented and discussed in the following publications Oracz et al., 2007a, 2008, 2009.
|
|
| |
 |
|
|

| |
Peer-reviewed publications |
 |
|
|
Oracz K., El-Maarouf-Bouteau H., Kranner I., Bogatek R., Corbineau F., Bailly C. (2009).
The mechanisms involved in seed dormancy alleviation by hydrogen cyanide unravel the role of reactive oxygen species as key actors of cellular signalling during germination.
Plant Physiology 150: 494-505.
Abstract PDF
Oracz K., El Maarouf-Bouteau H., Bogatek R., Corbineau F., Bailly C. (2008).
Release of sunflower seed dormancy by cyanide: crosstalk with ethylene signaling pathway.
Journal of Experimental Botany 59: 2241-2251.
Abstract PDF
Oracz K., El-Maarouf Bouteau H., Farrant J.M., Cooper K., Belghazi M., Job C., Job D., Corbineau F., Bailly C. (2007a).
ROS production and protein oxidation as a novel mechanism of seed dormancy alleviation.
The Plant Journal 50: 452-465.
Abstract PDF
Gniazdowska A., Oracz K., Bogatek R. (2007)
Phytotoxic effect of sunflower (Helianthus annuus L.) to hormonal balance (ABA : ethylene) in germinating mustard (Sinapis alba L.) seeds.
Allelopathy Journal 19: 215-226.
Oracz K., Bailly C., Gniazdowska A., Come D., Corbineau F., Bogatek R. (2007b).
Induction of oxidative stress by sunflower phytotoxins in germinating mustard seeds.
Journal of Chemical Ecology 33: 251-264.
Abstract PDF
Bogatek R., Gniazdowska A., Zakrzewska W., Oracz K., Gawronski S.W. (2006)
Allelopathic effects of sunflower extracts on mustard seed germination and seedling growth.
Biologia Plantarum 50: 156-158.
Gniazdowska A., Oracz K., Bogatek R. (2004).
Allelopathy - new interpretations of plant interactions.
Kosmos 53: 207-218.
|
|
Published contribution to academic conferences |
 |
|
|
Bogatek R., Oracz K., Gniazdowska A., (2005).
Ethylene and ABA production in germinating seeds during allelopathy stress.
In: Proceedings of the 4th World Congress on Allelopathy, Australia, International Allelopathy Society.
Oracz K., Bogatek R., Bailly C., Come D., Corbineau F., Gawronski S.W., (2003).
Allelopathic potential of sunflower. IV. Activation of antioxidative system in germinating mustard (Sinapis alba L.).
Acta Physiologia Plantarum 25: pp. 10.
Bogatek R., Gawronska H., Oracz K., (2003).
Involvement of oxidative stress and ABA in CN-mediated elimination of embryonic dormancy in apple.
In: The Biology of Seeds: Recent Research Advances – CABI Publishing, pp. 211-217.
|
|
|
|
Awards and distinctions |
 |
|
|
Alexander von Humboldt Fellowship – for the realization of postdoc in the Department of Molecular Plant Sciences, Albert Ludwigs University, Freiburg i. Br., Germany (2009).
The ISSS President’s Prize for the best Poster presentation by the title: “Signaling role of HCN and ROS in alleviation of sunflower embryo dormancy”. 9th ISSS Conference on Seed Biology, Olsztyn, Poland (2008).
Claude Leon Foundation Fellowship – for the realization of postdoc in the Department of Molecular and Cell Biology at University of Cape Town, Cape Town, South Africa (2008).
Distinction of the PhD degree entitled: "Role of hydrogen cyanide in dormancy removal of sunflower (Helianthus annuus L.) embryos", Warsaw/Paris, Poland/France (2008).
Award in the category - the best oral presentation and very interesting results presented at 3rd Conference of Polish Society of Experimental Plant Biology (Warsaw, Poland), by the title: “Possible interplay of HCN and ROS with ethylene signalling pathway in alleviation of sunflower embryos dormancy” (2007).
French Government Grant – for the realization of co-tutelle (Polish-French) thesis (PhD), French Embassy in Warsaw, Poland (2004).
Award of President of Warsaw Town – for one of the best students of Warsaw universities, Warsaw, Poland (2003).
III prize in the category - the best oral presentation by the title: “Allelopathic potential of sunflower. IV. Activation of antioxidative system in germinating mustard (Sinapis alba L.)”, 5th International Conference: Ecophysiological aspects of plant responses to stress factors, Cracow, Poland (2003).
Distinction of master degree by the title: “Oxidative stress in germinating mustard (Sinapis alba L.) seeds during allelopathy stress”, Warsaw, Poland (2003).
Bronze Award considered as one of the best posters presented in the III World Congress on Allelopathy, Tsukuba, Japan (2002).
|
|
|
|
Symposiums and conferences |
 |
|
|
Oracz K., El-Maarouf-Bouteau, H., Bogatek, R., Corbineau, F., Bailly, C., (6 – 11 July 2008).
“Signaling role of HCN and ROS in alleviation of sunflower embryo dormancy”. 9th ISSS Conference on Seed Biology, Olsztyn, Poland.
The ISSS President’s Prize for the best Poster presentation.
Bogatek R., Oracz K., Gniazdowska A., (19 – 22 Septemner 2007).
“Signaling role of ROS and phytohormones in allelopathics interactions between plants”. 7th International Conference – Eco-Physiological Aspects of Plant Responses to Stress Factors, Cracow, Poland.
Oracz K., El-Maarouf-Bouteau H., Bogatek R., Corbineau F., Bailly C., (26 – 30 August 2007).
“Possible interplay of HCN and ROS with ethylene signalling pathway in alleviation of sunflower embryos dormancy. 3rd Conference of Polish Society of Experimental Plant Biology, Warsaw, Poland.
Award in category the best oral presentation and the very interesting results.
Oracz K., El-Maarouf-Bouteau H., Bogatek R., Corbineau F., Bailly C., (1 – 4 July 2007).
“Unrevealing the molecular basis of HCN effect on alleviation of sunflower embryos dormancy: possible interplay with ethylene signalling pathway”. 2nd ISSS Workshop on Molecular Aspects of Seed Dormancy and Germination, Salamanca, Spain.
Bogatek R., Oracz K., Gniazdowska A., (14 – 18 May 2007).
„Allelochemicals as a signaling molecules in the negative plant-plant interaction”. 3rd International Symposium on Plant Neurobiology, Strebske Pleso, Slovakia.
Oracz K., Bailly C., Bogatek R., Corbineau F., (21 June 2006).
„Hydrogen cyanide in the regulation of germination of sunflower embryos”. Physiological and practical aspects of the yield and seed quality improvement by ecological methods. Seed Network Conference, Warsaw, Poland.
Oracz K., Bailly C., Corbineau F., (8 – 13 May 2005).
„Effect of cyanide on the germination of dormant sunflower (Helianthus annuus L.) seeds”. 8th International Workshop on Seeds – Germinating New Ideas, Brisbane, Australia.
Bogatek R., Oracz K., Gniazdowska A., (21 – 26 August 2005).
„Ethylene and ABA production in germinating seeds during allelopathy stress”. 4th World Congress on Allelopathy, Wagga Wagga, Australia.
Oracz K., Gniazdowska A., Corbineau F., Come D., Skoczowski A., Janeczko A., Bogatek R., (3 – 5 June 2004). „Comparison between allelopathy and water stress in germinating mustard seeds”. 2nd European Allelopathy Symposium, Pulawy, Poland.
.
Bailly C., Mamadou N., Oracz K., Come D., Corbineau F., (24 – 28 May 2004).
„Is there a role for cyanide in sunflower seed germination”. 3rd International Symposium on Plant Dormancy, Wageningen, Netherlands.
Oracz K., Bogatek R., Bailly C., Come D., Corbineau F., (29 April – 4 May 2004).
“Physiological and biochemical responses of mustard seeds to allelopathy stress. I. Induction of oxidative stress”, An International Meeting on Seeds and the Environment, Rhodes Island, Greece.
Oracz K., Bogatek R., Bailly C., Come D., Corbineau F., (23 – 25 October 2003).
“Oxidative burst in mustard seeds as a consequence of allelopathic stress induced by
sunflower.” International Workshop on Applied Seed Biology: New Developments in Seed Quality Improvement, Lodz, Poland.
Oracz K., Bogatek R., Bailly C., Come D., Corbineau F., Gawronski S. W., (15 – 17 September 2003).
“Allelopathic potential of sunflower. IV. Activation of antioxidative system in germinating mustard (Sinapis alba L.)”, 5th International Conference: Ecophysiological aspects of plant responses to stress factors, Cracow, Poland.
III prize in category the best oral presentation.
Oracz K., Bogatek R., (3 – 6 September 2003).
„Oxidative burst in germinating mustard seeds during allelopathy stress”, 1st Conference of Polish Society of Experimental Plant Biology, Olsztyn, Poland.
Bogatek R., Oracz K., Bailly C., Gawornska H., Come D., Corbineau F., Gawronski W. (26 – 30 August 2002).
„Induction of oxidative stress by sunflower allelopathics during germination of mustard (Sinapis alba L.) seeds” . III World Congress on Allelopathy, Tsukuba, Japan.
Bronze Award considered as one of the best posters presented in the Congress.
|
|
|
|
Invited seminars |
|
Oracz K., Corbineau F., Bailly C. (7 February 2007).
ROS production and protein oxidation as a novel mechanism of seed dormancy alleviation - Royal Botanic Gardens, Wakehurst Place, Kew, United Kingdom.
|
|
| |
|
| |
 |
|
|
| |
 |
|
|
|
|
 |
| |
|
|
|

|
 |

